The CBSE board has made the Class 10 Science Additional Practice Questions Paper 2024 available for download and practice at www.cbseacademic.nic.in.
For Class 10 students in the CBSE curriculum, the Science exam holds a significant place. To help students excel in their Science papers, CBSE has compiled a list of additional practice questions for the year 2024.
Why is Class 10 CBSE Science Additional Practice Questions 2024 Essential?
Class 10 CBSE Science Additional Practice Questions are key to success in any examination. It not only helps you grasp the concepts better but also boosts your confidence. Regular practice makes you familiar with different types of questions that may appear in the board exams.
Get Prepared for CBSE Class 10 Science 2024 with Additional Practice Questions
Get ready to excel in the CBSE Class 10 Science exam in 2024 with CBSE-released additional practice questions. These meticulously crafted questions cover a diverse range of topics, from Physics to Chemistry and Biology.
They’re designed to align with the CBSE syllabus, helping students gain a deep understanding of crucial concepts. This Class 10 CBSE Science Additional Practice Questions not only boosts students’ confidence but also prepares them for various question types that may appear in the exam.
Whether students are struggling with complex subjects or aiming for a top score, these Class 10 CBSE Science Additional Practice Questions are the key to success. Don’t miss out – access them now and pave your way to excellence.
Related Topics
General Instruction of Class 10 CBSE Science Questions 2024
This Additional Practice Science Questions paper consists of 39 questions in 5 sections.
All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
1. Section A consists of 20 objective-type questions carrying 1 mark each.
2. Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
3. Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words.
4. Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
5. Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
Download Class 10 CBSE Science Additional Practice Questions Papers with solutions
Download the CBSE Class 10 Science Practice Questions and Solutions PDF for 2024 and previous years pave your way to success. This resource is your ultimate guide, offering comprehensive coverage of the syllabus and detailed solutions. Achieve excellence in your Science exam with this essential tool at your fingertips.
| 2023-2024 | Science Practice Questions | Solutions |
| 2022-2023 | Science Practice Questions | Solutions |
| 2021-2022 | Science Practice Questions | Solutions |
Explore Class 10 CBSE Science Additional Practice Questions and Solutions 2024
CBSE
Science (086)
Class X 2023-24
Maximum marks: 80 Time Allowed: 3 hours
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1 – 20. There is no negative mark for incorrect response.
1. A single displacement reaction is represented below.
PQ+R —-> PR + Q
Which of the following is true about the reactants and products?
| Option | Type of ion of R in product | Stability of PR as compared to PQ |
| A | cation | more stable |
| B | cation | less stable |
| C | anion | more stable |
| D | anion | less stable |
(a) A
(b) B
(c) C
(d) D
Ans: (c) C
2. Some types of chemical reactions are listed below.
– decomposition
– combination.
– displacement
– double displacement
Which two of the following chemical reactions are of the SAME type?
P) AgNO3 + NaCl —> AgCl + NaNO3
Q) Mg + 2 HCl —> MgCl₂ + H₂
R) CH4 + 2 O2 —> CO2 + 2 H₂O
S) 2 KOH + H₂SO4 —> K₂SO4 + H₂O
(a) P and Q
(b) Q and R
(c) R and S
(d) P and S
Ans: (d) P and S
3. Neetu has two test tubes containing dilute hydrochloric acid and dilute sodium hydroxide solution, but they are not labeled. Adding which of the following solutions to the test tubes will help her visually identify the acidic and basic solution?
(a) only vinegar
(b) only baking soda
(c) only sodium chloride
(d) either vinegar or sodium chloride
Ans: (b) only baking soda
4. Sonia has aqueous solutions of three salts, sodium acetate, sodium chloride and ammonium chloride in three test tubes. The test tubes are not labeled. On checking, she finds the pH of the solutions to be 4.6, 7.0 and 8.9.
Which of the following correctly matches the salts with their respective pH?
| pH 4.6 | pH 7.0 | pH 8.9 | |
| A | sodium acetate | sodium chloride | ammonium chloride |
| B | sodium chloride | ammonium chloride | sodium acetate |
| C | ammonium chloride | sodium acetate | sodium chloride |
| D | ammonium chloride | sodium chloride | sodium acetate |
(a) A
(b) B
(c) C
(d) D
Ans: (d) D
5. Galvanisation is a process of coating iron articles with a layer of zinc to prevent the iron from rusting. The iron is protected even if the zinc coating is scratched and iron is exposed.
Which of the following is true about how zinc prevents the rusting of iron?
P) A galvanised iron article does not undergo oxidation.
Q) The zinc coating prevents contact of iron with air.
R) Zinc undergoes corrosion more easily than iron.
(a) only P
(b) only Q
(c) only P and Q
(d) only Q and R
Ans: (d) only Q and R
6. During purification of a metal by electrolysis, what happens at the negative electrode?
(a) Metal ions lose electrons to become neutral atoms.
(b) Neutral metal atoms gain electrons to become ions.
(c) Neutral metal atoms lose electrons to become ions.
(d) Metal ions gain electrons to become neutral metal atoms
Ans: (d) Metal ions gain electrons to become neutral metal atoms
7. Metals are lustrous and shine especially when their freshly cut surfaces are Exposed.
Salma cut pieces and compared the lustre of the freshly cut surfaces of the following metals. aluminium, sodium, copper, iron The freshly cut surface of which of these metals is likely to lose its lustre first on exposure to air?
(a) aluminium
(b) sodium
(c) copper
(d) iron
Ans: (b) sodium
8. Which of the following statements is TRUE about the uptake of water in plants?
(a) It occurs all the time due to diffusion.
(b) Water enters the roots due to osmosis.
(c) At night when transpiration is low, roots do not take up water.
(d) The movement of water from roots to leaves is bidirectional.
Ans: (b) Water enters the roots due to osmosis.
9. Oxygen saturation levels refer to the extent hemoglobin is bound to oxygen. As altitude increases, the atmospheric pressure decreases. Which of the following graphs correctly represents the oxygen saturation levels as altitude increases?

(a) P
(b) Q
(c) R
(d) S
Ans: (b) Q
10. Which of the following method/s are useful to prevent fertilisation even when ovulation occurs?
P) surgical blocking of the fallopian tube
Q) copper-T
R) oral pills
S) condom
(a) only P
(b) only Q and R
(c) only P, Q and S
(d) only Q, R and S
Ans: (c) only P, Q and S
11. In cattle, having horns is a recessive trait (h) to not having horns (H). When cattle with horns are crossed with cattle that do not have horns, the number of offspring having horns was equal to those not having horns.
Which of the following is MOST LIKELY to be true?
(a) Both parents are homozygous dominant.
(b) One parent is homozygous dominant.
(c) Both parents are heterozygous.
(d) One parent is heterozygous.
Ans: (d) One parent is heterozygous.
12. Patient X was suffering from a pancreatic condition due to which the pancreas was not functioning adequately. Which of the following is a doctor likely to suggest to such an individual?
(a) including a large amount of protein in the diet
(b) eating a diet with low-fat content
(2) eating only carbohydrates
(d) including only liquid foods
Ans: (b) eating a diet with low-fat content
13. When an object was kept at position X in front of a concave mirror, an enlarged and virtual image was formed. Which among the following identifies ‘X’ correctly?
Ans: (b) anywhere between the pole and principal focus
14. The face of the moon that is visible to us is called as the near side and the face of the moon which is invisible to us is called as far side. What colour would the sky appear to an astronaut standing on the “far side” of the Moon and why?
(a) blue, as the Moon’s atmosphere scatters sunlight just like Earth
(b) white, as the Moon’s surface reflect all the light that falls on it
(c) black, as there is no atmosphere on Moon to scatter sunlight
(d) black, as sunlight does not fall on the far side of the Moon
Ans: (c) black, as there is no atmosphere on Moon to scatter sunlight
15. Plants receive energy from the Sun which they utilise for several processes. The energy utilized for which of the following plant processes gets transferred to the next trophic level that consumes plants?
(a) only growth
(b) only respiration
(c) only transport of substances and reproduction
(d) all – growth, photosynthesis, respiration and transport of substances
Ans: (a) only growth
16. The action of which among the following is crucial to the formation of ozone?
(a) humans
(b) sunlight
(c) carbon dioxide
(d) chlorofluoro carbons
Ans: (b) sunlight
Question No. 17 to 20 consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
a) Both A and R are true, and R is the correct explanation of A.
b) Both A and R are true, and R is not the correct explanation of A.
c) A is true but R is false.
d) A is false but R is true.
17. Assertion (A): Zinc oxide can be reduced to zinc metal on heating with carbon.
Reason (R): Carbon is less reactive than zinc.
Ans: (c) A is true, but R is false.
18. Assertion (A): Variations always provide a survival advantage to an organism.
Reasons (R): Variations can be caused due to incorrect DNA copying.
Ans: (d) A is false and R is true.
19. Assertion (A): Iron filings scattered around a straight current-carrying conductor in a plane perpendicular to the length of the conduit themselves in concentric circles.
Reason (R): Magnetic field has both magnitude and direction.
Ans: (b) Both A and R are true, and R is not the correct explanation of A.
20. Assertion (A): Omnivores receive 10% of their energy from the trophic level 1 below them.
Reason (R): An omnivore is always in the trophic level just above
Herbivores.
Ans: (c) A is true but R is false.
Section B
Question No. 21 to 26 are very short answer questions
21. (a) Write the balanced chemical equation for the reaction that is prevented by storing potassium metal under kerosene.
(b) Identify the type of chemical reaction that is prevented.
Ans: (a) 4 K + O2 —> 2 K2O [1 mark] (b) combination reaction OR oxidation reaction OR redox reaction
22. Ravi cultivated mustard, a plant with bisexual flowers, on his farm. His plants were diseased due to a gene defect and therefore had reduced yield. Ravi removed the stamens from the diseased plants and also planted fresh disease- free mustard plants where he removed the pistils.
Ans:
1 mark each for the following:
- Ravi made the bisexual flower unisexual thereby encouraging crosspollination instead of self-pollination.
- Cross-pollination will increase variation and thereby the chances of having
more disease-free offspring.
[Accept any other valid answer.]
23. How will Ravi’s strategy help in improving the yield of mustard? A plant X was enclosed in a glass jar with some lizards. A similar plant Y was enclosed in another glass jar but without lizards. Both the jars are kept under the same light conditions for a few hours. Which plant is likely to photosynthesize more and why?
OR
Proteinuria is a condition in which significant amounts of protein can be detected in urine. Which process in the nephron is likely to be affected causing proteinuria? Justify.
Ans:
1 mark each for the following:
- Plant X
- Due to respiration of the lizard, the amount of carbon dioxide will increase
leading to a higher amount of photosynthesis.
OR
1 mark each for the following:
- Filtration OR selective reabsorption by the nephron may not be
functioning properly. - Improper filtration will lead to proteins getting filtered even though they
are not waste.
OR
After filtration, useful substances such as proteins may not be getting
reabsorbed.
24. Search mirrors are mirrors that are used to look for hidden objects underneath the cars as shown. The hidden objects can be easily spotted as the mirror provides a wider field of view.

(a) What type of mirrors are generally used to make search mirrors?
(b) With the help of a ray diagram describe the nature of image formed by the type of mirror identified in (a).
Ans:
(a) convex mirror [0.5 marks]
(b)
- 0.5 marks for any two of the following:
virtual, erect and diminished - 1 mark for the ray diagram

25. Ramya wants to measure the current flowing through the circuit shown below.
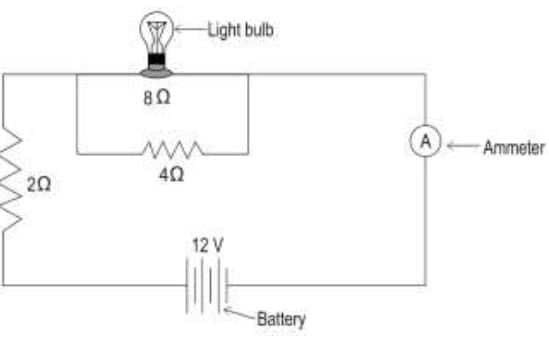
Which among the four ammeters can she use for the same? Show your calculations.
| Ammeter | Minimum range | Maximum range |
| P | 0 mA | 1 m A |
| Q | 0 mA | 10 mA |
| R | 0 A | 1 A |
| S | 0 A | 10 A |
OR
A helical coil whose length is greater than its diameter is connected to a battery as shown below.

(a) How does the magnetic field at point P compare with the magnetic field at point Q? Justify your answer.
(b) State one way in which the strength of the magnetic field inside a current-carrying helical coil can be changed?
Ans:
- From Ohm’s law we have
V = IR
I = V/R
given V = 12 V and R1 = 2 ohm, R2 = 8 ohm and R3 = 4 ohm
Therefore,
net resistance R = R1 + (R2 x R3)/ (R2 + R3)
= 2 + (8 x 4/8 + 4)
= 2 + 32/12
= 2 + 2.66
R = 4.66 ohm [1 mark] - I = 12/4.66
I = 2.58 A [0.5 marks]
- She can use ammeter S to measure the current in the circuit. [0.5 marks]
OR - The magnetic field at P and Q is the same. [0.5 marks]
- because the magnetic field lines inside the helical coil of wire which
behaves like a solenoid is uniform/in the form of parallel straight lines. [0.5
marks]
[Accept any other valid another answer.]
(b) 1 mark for any one of the following: - increasing/decreasing the number of turn in the coil
26. Shown Below are two food pyramids.

The pyramid representing the land ecosystem is traditional with producers being greater in mass than primary consumers and so on. Sometimes, in aquatic ecosystems, an inverted pyramid exists. Here, the total mass of producers (phytoplankton) is much smaller than the top consumers (big fishes).
(a) Which level is likely to have the most amount of energy in such an aquatic ecosysten? Give a reason to support your answer
(b) Such aquatic ecosystems are not considered to be sustainable. Justify this Statement.
Ans:
(a) 0.5 marks each for the following:
- phytoplankton
- Producers will still have the highest amount of energy captured from
sunlight which will continue to reduce as we move towards the top of the
pyramid.
[Accept any other valid answer.]
(b) Since the total mass in the lower trophic level is lesser there will be
lesser food available to higher trophic levels causing organisms to die
sooner than usual. [1 mark]
[Accept any other valid answer.]
Section C
Question No. 27 to 33 are short answer questions
27. Equal sized bars of aluminium and iron are exposed to the environment as shown below.

Which of them is likely to corrode till the level marked by the line FIRST?
Justify your answer.
Ans:- The iron bar will corrode till the level marked by the line first. [1 mark] – Iron gets oxidised on exposure to air and moisture. The layer of rust formed on the surface allows air and moisture to pass through and reach the metal, causing corrosion to continue. [1 mark] – Aluminium gets oxidised on exposure to air. The layer of oxide formed on the surface forms a protective coating that prevents air from reaching the metal and thus prevents further corrosion. [1 mark]
28. The electronic configuration of some elements is given in the table below.
| Element | Electronic configuration |
| P | 2, 8, 8 |
| Q | 2, 8, 8, 1 |
| R | 2,6 |
| S | 2,5 |
| T | 2, 8, 2 |
| U | 2, 8, 7 |
(a) Identify any two pairs of cements that will react to form compounds by a transfer of electrons.
(b) Write the molecular formula of the compounds formed by the pairs of elements identified in (a).
OR
Ar tal X is obtained from its chloride salt by exposure to sunlight.
(a) In which section of the reactivity series of metals- top, middle or bottom, is it likely to be placed? Justify your answer.
(b) Identify the type of reaction the chloride salt of metal X undergoes on exposure to sunlight.
Ans:
(a) 1 mark each for any two correct pairs of elements identified such as:
- Q and R
- Q and U
- T and R
- T and U
(b) 0.5 marks each for the formula for two compounds such as: - Q2R
- QU
- TR
- TU2
OR
(a) – bottom section [1 mark] - Metals at the bottom of the reactivity series are the least reactive. They
occur in their free state; their compounds are unstable and hence easily
converted to metal. [1 mark]
(b) photolytic decomposition [1 mark]
29. In animals, hormones can be secreted by one organ and can act on multiple organs. Justify this statement by explaining the effect of a single animal hormone on three organs.
Ans:
1 mark each for the following:
- Adrenaline induces the sweat glands to produce more sweat.
- It acts on the heart to increase the contraction of its muscles/pumping
causing improved oxygen delivery. - It acts on blood vessels of the digestive system constricting them.
[Accept any other valid answer.]
30. If two pea plants having round and green seeds (RRGg) are crossed, identify the percentage of the following with respect to the F1 generation:
(a) gametes having both the round and yellow seed traits
(b) offspring having the same genotype as the parents
(c) offspring having the same phenotype as the parents
Ans:
1 mark each for the following:
(a) 50%
(b) 50%
(c) 75%
31. Absolute refractive indices of two media P and Q are 1.33 (np) and 2.52 (no) respectively. The speed of light in medium P is 2 x 10 m/s.
(a) What would be the speed of light in medium Q (Vo)?
(b) If the angle of incidence for a ray of light travelling from medium P to Qis
0°, then what will be the path of light in the medium Q?
Ans:
(a)
nP = (Speed of light in vacuum/ speed of light in medium P)
nq = (Speed of light in vacuum/ speed of light in medium Q)
[0.5 marks]
Therefore,
nP/nQ = VQ/VP
= 1.33/2.52 = VQ / 2 x 108 [0.5 marks]
VQ = (1.33 x 2 x 108)/ 2.52
= 1.056 x 108m/s [1 mark for the calculation and arriving at the correct
answer.]
(b) the ray will travel undeviated through the medium Q [1 mark]
[Accept any other valid answer.]
32. Kaveri conducted an experiment to study the energy efficiency of different bulbs. She connected a bulb A having a resistance of 100 ohms to a 240 V power supply in a laboratory.
(a) How much energy will be consumed by the bulb, if it is kept ON for 4 hours each day for a week? Express your answer in kJ.
(b) Kaveri connects another similar bulb B in series with bulb A and connects the combination to a 240 V supply. Will there be any change in the brightness with which bulb A glows now? Explain mathematically.
Ans:
(a) Given V = 240 V and R = 100 ohms
Therefore,
Power (P) = V2/R
= (240)2/100
= 576 W [0.5 marks]
Energy consumed by bulb A = P × t
E = 576 x 4 x 7 × 60 × 60
E = 58,060.8 kJ [0.5 marks]
[Marks to be awarded if the students use any other method to arrive at the
correct answer.]
(b) When bulbs A and B are connected in series:
Rnet = R1 + R2
= 100 + 100
Rnet = 200 ohms
Total power consumed by bulb A when connected in series with bulb B
Ptot = V2/Rnet = (240)2/200 = 288 W [0.5 marks]
PA’ = Ptot/2 = 144 W [0.5 marks]
Power consumed by bulb A when connected without bulb B to 240 V
PA= V2/R
= (240)2/100
= 576 W
As PA’ < PA , the brightness of the bulb A decreases when connected in series with bulb B. [1 mark] [Marks to be awarded if the students use any other method to arrive at the correct answer.]
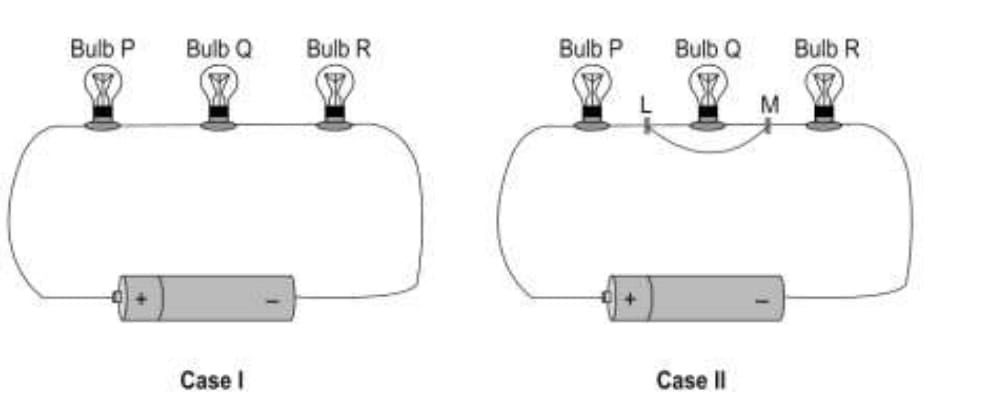
33. (a) Vijaya connects three bulbs P, Q and R is series with a battery in two different ways using identical conducting wires as shown below. She notices that in case I all three bulbs glow but in case II only the bulbs P and R continue to glow. What could he reason for the bulb Q to not glow in case II? Explain.
(b) Two resistances when connected in parallel give a combined resistance of 10/3 ohms. When the same two resistors are connected in series, the combined resistance becomes 15 ohms. Calculate the individual resistance of each resistor.
Ans:
(a) The current will flow through the additional wire that connects the
points L and M (avoiding the bulb) as it offers a path of least/lower
resistance as compared with the bulb [1 mark]
(b)
3/10 = 1/R1 + 1/R2 ………………. (1)
R1 + R2 = 15, R1 = 15 – R2
Substituting in (1)
3/10 = (15 – R2 + R2)/ (15 – R2) R2 [1 mark]
15R2 – R2
2 = 150/3 = 50
R2
2 – 15 R2+50 = 0
R2 = 10 ohm, R1 = 5 ohm
or
R1 = 10 ohm, R2 = 5 ohm
[1 mark]
[Accept any other correct method]
Section D
Question No. 34 to 36 are long answer questions.
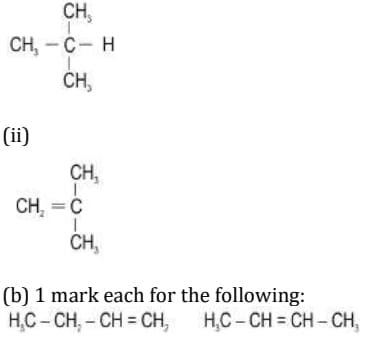
34. A carbon compound P has six carbon atoms and twelve hydrogen atoms.
(a) Is P a saturated or unsaturated carbon compound. Justify your answer by drawing the structural formula.
(b) Describe a test that can be used to determine if compound P is saturated or unsaturated.
(c) Name the products that are formed on burning compound P in excess of air.
OR
A carbon compound P is found to be neutral when tested with red and blue litmus. A gas, that burns with a ‘pop’ sound, is produced when a metal reacts with carbon compound P.
(a) Write the chemical equation for the reaction.
(b) The carbon compound P is heated with concentrated sulphuric acid to produce carbon compound Q.
(i) Write the chemical equation for the reaction.
(ii) Describe the type of flame that Q produces on combustion.
(c) What is likely to be observed on heating compound P with ethanoic acid with an acid as catalyst? Write the chemical equation for the reaction.
Ans: (a) Compound P may be either saturated or unsaturated. [1 mark] – saturated compound: cyclohexane [1 mark]

[Accept any correct structural isomer]
(b) burning the compound in an excess of air will produce a sooty flame if it is unsaturated and a clean flame if it is saturated [1 mark] (c) carbon dioxide and water [1 mark] [No marks to be given for only one product]
OR
(a) 2 Na + 2 CH3 – CH2OH —> 2 CH3 – CH2O-Na+ + H2 [Marks to be given for writing the correct reaction of sodium metal with any alcohol.]
(b) (i) CH3 – CH2OH —> CH2 = CH2 + H2O [1 mark] (ii) Compound Q (ethylene) burns with a yellow flame with black smoke. [1 mark]
(c) A compound with a fruity smell will be produced. [1 mark]

35. (a) Certain specialised cells in animals called stem cells have the ability to divide and differentiate into different cell types. This helps in the replacement of a damaged organ.
Name and explain two methoss fasexual reproduction that are similar to stem cells and occur mostly in multicellular organisms.
(b) Identify TWO pairs of reproductive organs in males and females that a fitnctionally similar to each other. Justify.
OR
(a) Sagar saw a beautiful rose and smelled it. As he was smelling it, he happened to touch a thorn and pull his hand away. State TWO differences and similarities cach in the way the nervous system performs the two actions.
(b) Are all involuntary actions reflex actions? Justify.
Ans:
0.5 marks each for the name and 0.5 marks each for the explanation:
- Regeneration
- In this process, if an individual organism is cut or broken up into many
pieces, many of these pieces grow into separate individuals. - Budding
- In budding, a small outgrowth or bud forms on the parent organism, which
eventually detaches and develops into a new individual.
[Accept any other valid answer.]
(b)
- testes and ovaries [0.5 marks]
- Both structures perform the function of producing gametes and hormones
crucial for reproduction [1 mark] - vas deferens and fallopian tube [0.5 marks]
- Both structures are responsible for carrying the gamete to the site of
fertilisation. [1 mark]
[No marks are to be allotted if the pair mentioned is incorrect.]
OR
(a) 1 mark for each point such as:
Similarities: - In both cases, the signal is initiated by receptors located at the specific
sense organ. - In both cases, neurotransmitters are released and accepted by neurons to
carry the impulse.
Differences: - The action of smelling the rose is voluntary whereas pulling the hand
away is involuntary in nature. - While smelling the rose, the nerve impulse reaches the brain and back
whereas on touching a thorn the nerve impulse travels only to the spinal
cord and back.
[Accept any other valid points.]
(b) 0.5 marks each for the following: - No
- Involuntary actions occur with or without a stimulus whereas reflex
actions always need a stimulus.
[Accept any other valid answer.]
36. Savera passed a beam of white light through a series of equilateral prisms as shown.

(a) What colour(s) will be seen on the screen?
(b) Copy the diagram above and draw the beam entering Prism 1 and emerging from Prism 3 and falling on the screen.
(c) Name all the processes that takes place when the beam of light enters the Prism 1 and emerges from Prism 3.
OR
(a) Rupal suffers from myopia. Where would the image form in her eye?
(b) Name the type of lens that is generally used to correct myopia.
(c) Rupal underwent cataract surgery and her eye lens was replaced with an artificial lens with a fixed focal length, made of a plastic material, silicone. State one likely visual disadvantage that Rupal is likely to experience as compared to a person who has normal eyesight.
(d) Identify the parts of the eye labeled in the diagram from the descriptions given below by writing the labels as your answer.

i) It helps in changing the focal length of the lens.
(ii) causes most of the refraction of the light entering the eye.
(iii) t controls the amount of light entering the eye.
(iv) It acts as a screen on which the image is formed.
Ans:
(a) VIBGYOR [1 mark]
- violet
- indigo
- blue
- green
- yellow
- orange
- red
(b) 1 mark each for drawing the incident and emergent rays in the three
prisms respectively.
[Image not up to scale.]

(c) 0.5 marks each
- dispersion
- refraction
OR
(a) in front of the retina [1 mark]
(b) concave lens [1 mark]
(c) The person would not have the power of accommodation. [1 mark]
(d) 0.5 marks each for the following:
(i) R
(ii) Q
(iii) P
(iv) S
Section – E
Question No. 37 to 39 are case-based/data -based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
37. Given below is a four carbon skeleton of a hydrocarbon compound.

(a) Fill in the hydrogen atoms/bonds to form:
(i) a saturated hydrocarbon
(ii) an unsaturated hydrocarbon.
(b) If the four-carbon skeleton is of a straight chained alkene, draw the structures of all the possible compounds.
OR
If the four-carbon skeleton is of a straight chained alkyne:
(i) How many carbon atoms may NOT be bonded to any hydrogen atoms?
(ii) How many hydrogen atoms will there be in the compound?
Ans: (a) 1 mark each for the following:
(i)
OR
(i) one or two [1 mark]
(no marks if both 1 or 2 not given)
(ii) six [1 mark]
38. Fam and Asha were a married couple. They had four children – one of these children had sickle cell anemia whereas the other four children did not show symptoms. Ram and Asha did not show symptoms of sickle cell anaemia. The trait for sickle cell anaemia is not linked to the sex chromosomes.
(a) Is this disease caused by a dominant or recessive trait? Why?
(b) If the child that had sickle cell anaemia got married to a person without a mutation in the sickle cell anaemia gene, what percentage of their children would have sickle cell anaemia? Show the cross.
OR
Identify the genetic composition of the sickle cell anaemia trait in Asha and Ram and use that to predict the genetic composition in the other four children who did not show symptoms that Ram and Asha had.
Ans:
(a) 1 mark for each of the following:
- It is recessive.
- If it was dominant, it would have appeared in more than 1 child.
[Accept any other valid answer. No marks to be awarded if the correct reason
is not mentioned.]
(b) 1 mark for the cross and 1 mark for the percentage:

- None of the children would have sickle cell anaemia/0% children would
have sickle cell anaemia.
OR
Ram’s genetic composition: Ss [0.5 marks]
Asha’s genetic composition: Ss [0.5 marks]
Four possible genetic compositions in children: SS, Ss, Ss, ss. [0.5 marks]
So, the composition of the other three children would either be Ss or ss. [0.5
marks]
39. Four resistors, a voltmeter and a battery are connected in a circuit as shown below.

(a) What is the net resistance in the circuit?
(b) How much potential difference will the voltmeter connected across the resistor R4 measure?
OR
What is the power dissipated by the resistor R₁?
(c) If R3 is removed, will the net current in the circuit increase or decrease or remain the same? Justify your answer.
Ans:
(a) The net resistance is: R1 + (1/R2 + 1/R3) + R4 [0.5 marks]
= 15 + 10 + 15
R = 40 Ω [0.5 marks]
(b) Voltage drop across R4 = Net current x R4
Net current = V/R
= 20/40
= 0.2 A [1 mark]
Voltage drop ac
OR
Power dissipated by the resistor R1 is given by:
P = I2R1
I = V/R
= 20/40
I = 0.2 A [1 mark]
Therefore,
Power = (0.2)2 x 15
= 0.6 W [1 mark]
(c)
- net current will decrease [0.5 marks]
- because R3 is connected in parallel and removing it will increase the net
resistance in the circuit thereby reducing the net current. [0.5 marks]
[Accept any other valid correct answer.]
Related Topics







